Oral GnRH Antagonists A Significant Advancement in Endometriosis and Adenomyosis Treatment
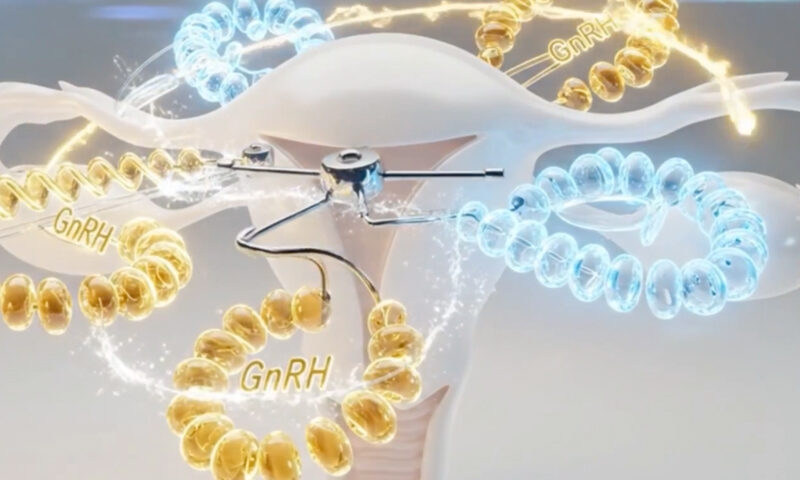
Oral GnRH antagonists have become an important advancement in the medical management of endometriosis and are increasingly being explored for adenomyosis. These agents work by directly blocking GnRH receptors in the pituitary gland, producing an immediate decrease in LH and FSH and therefore suppressing ovarian estrogen production without causing the “flare effect” seen with older GnRH agonists.